Featured
What Ions Are Present In Hclo4
What Ions Are Present In Hclo4. What iaons are present when hclo4 is dissolved in water? 2) identify the precipitate (if any) that forms when the following solutions are mixed, and write a balanced equation for each reaction.
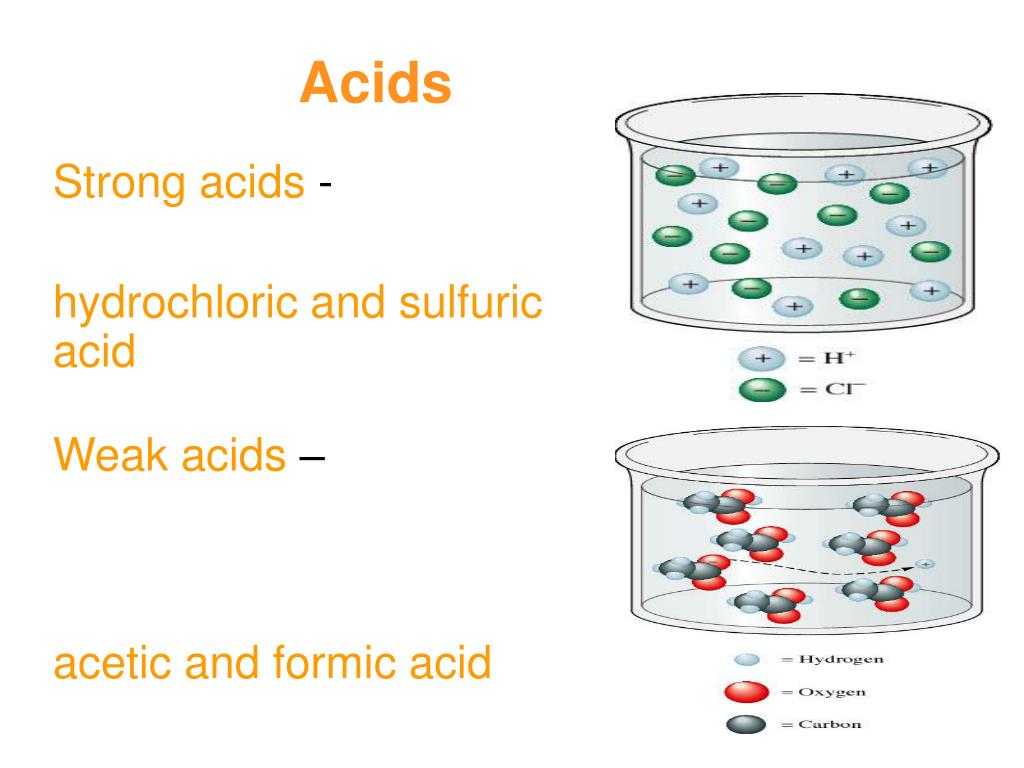
Acids create hydrogen (h+) ions when dissolved in water.bases create. Specify what ions are present in solution upon dissolving each of the following substances in water. Cations are ions with a positive charge.
Express Your Answers Separated By A Comma.
Dissolution is the process of in which the solute, dissolves and breakdowns into a solvent to form…. 2) identify the precipitate (if any) that forms when the following solutions are mixed, and write a balanced equation for each reaction. Anions are ions with a.
The Bond May Result From The Electrostatic Force Of Attraction Between Oppositely Charged Ions As In.
Does hclo4 dissociate in water? Mgcl2 k2co3 hclo4 nach3coo we don’t have your requested question, but here is a suggested video that might help. The ions present in the solution upon dissolving h c l o x 4 \\ce{hclo4}.
Specify What Ions Are Present In Solution Upon Dissolving Each Of The Following Substances In Water.….
Specify what ions are present in solution upon dissolving each of the following substances in water. Calculate the formal charge on the all atoms present in the molecules n o 3 −. Specify what ions are present in solution upon dissolving each of the following substances in water.
Hclo4 ( Perchloric Acid ) Is An Ionic Bond.
1) specify what ions are present upon dissolving each of the following substances in water: We review their content and use your feedback to keep the quality high. Acids create hydrogen (h+) ions when dissolved in water.bases create.
Assume Each Compound Is Dissolved In Water.
It is a conjugate acid of a perchlorate. Hcl hbr hi hclo 3 hclo 4 hno 3 and h 2 so 4). Specify what ions are present in solution upon dissolving each of the following substances in water:
Popular Posts
Blender Ascii Fbx Files Are Not Supported
- Get link
- X
- Other Apps
Comments
Post a Comment